be controlled
throughout
the dive




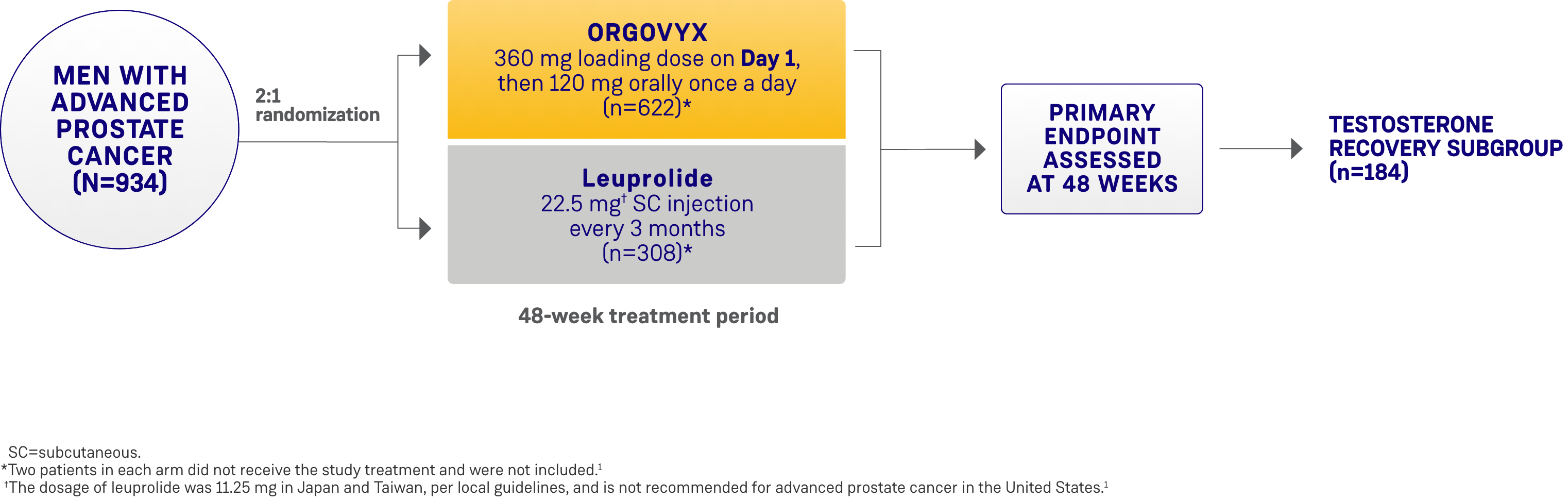
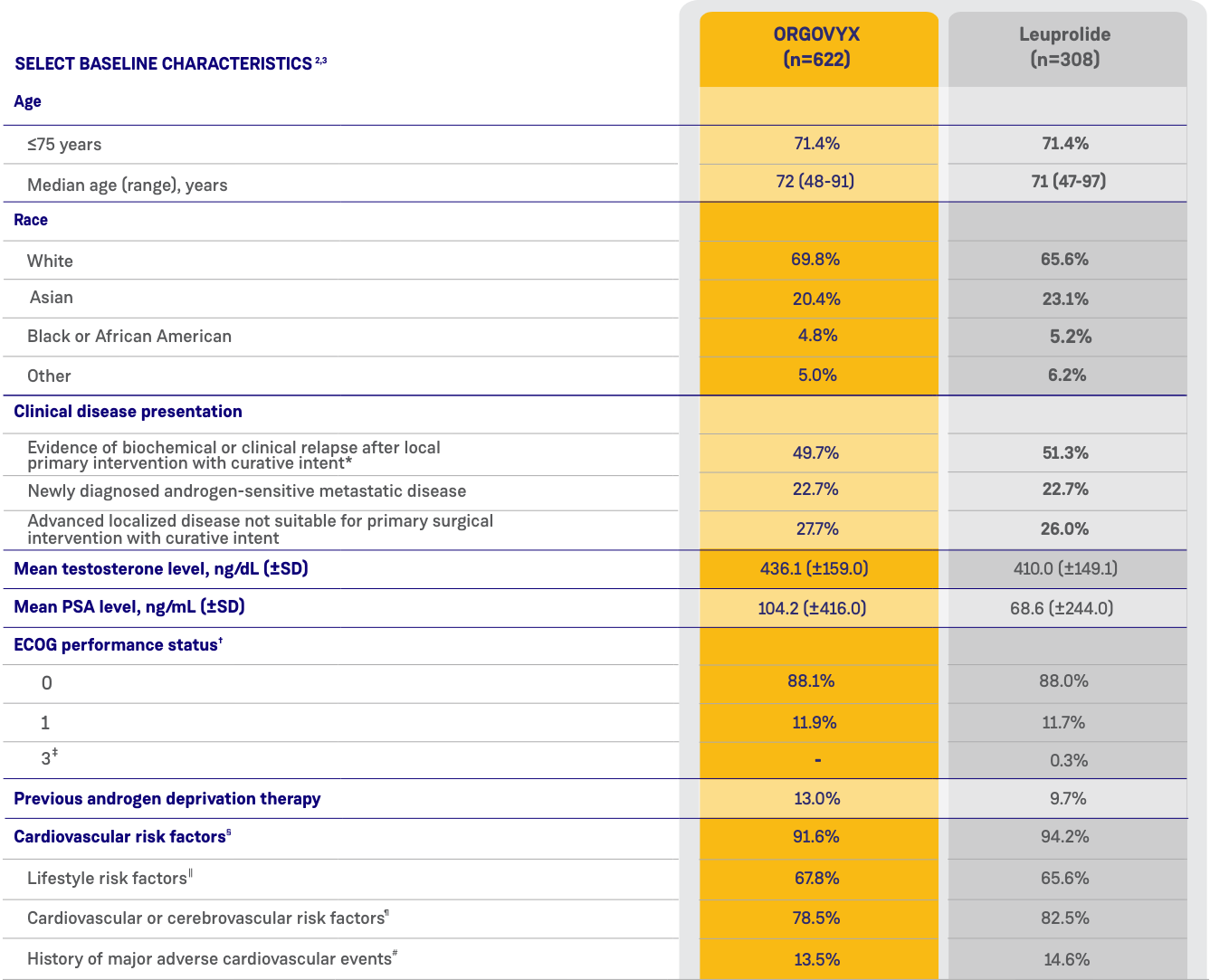


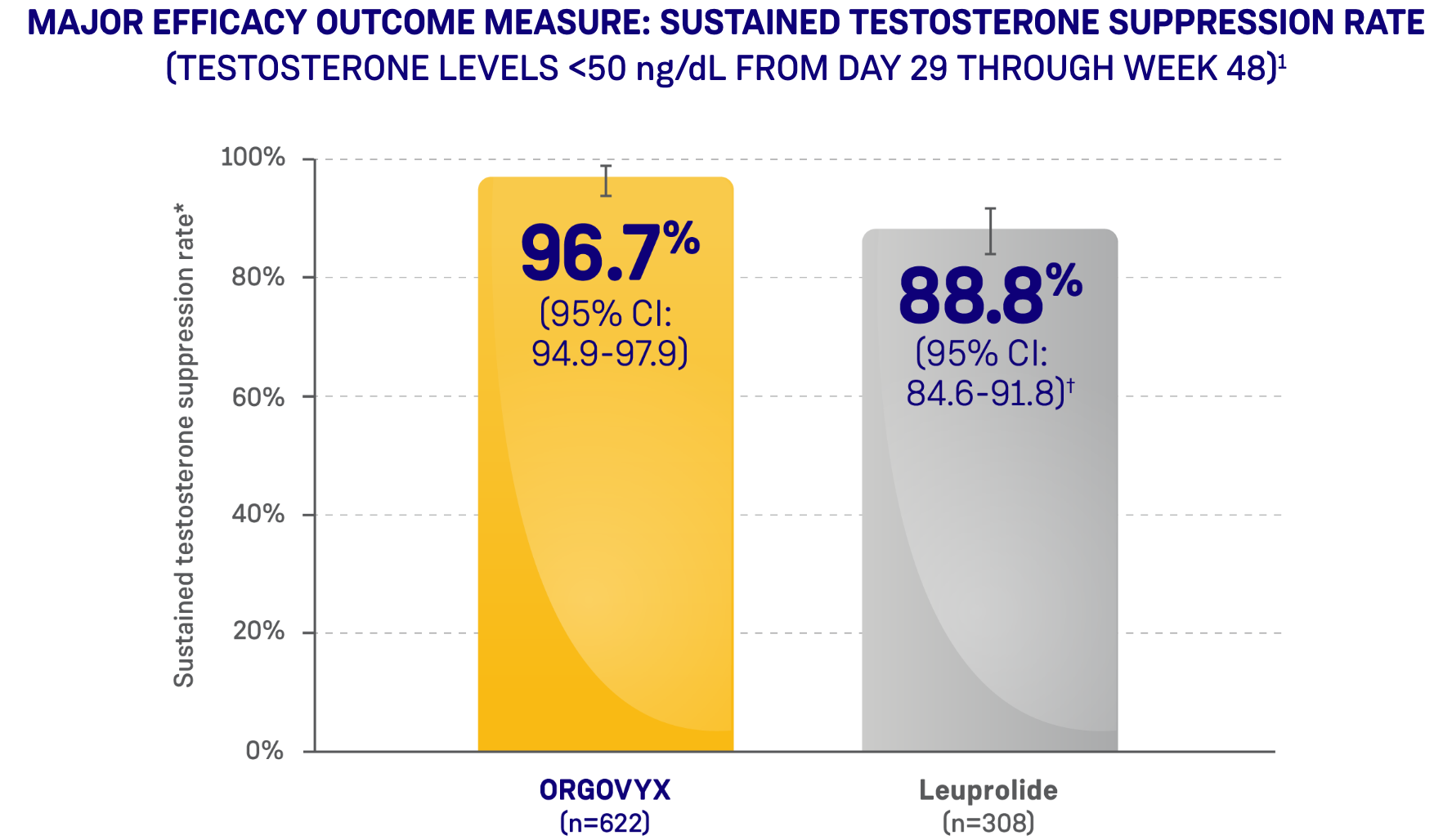
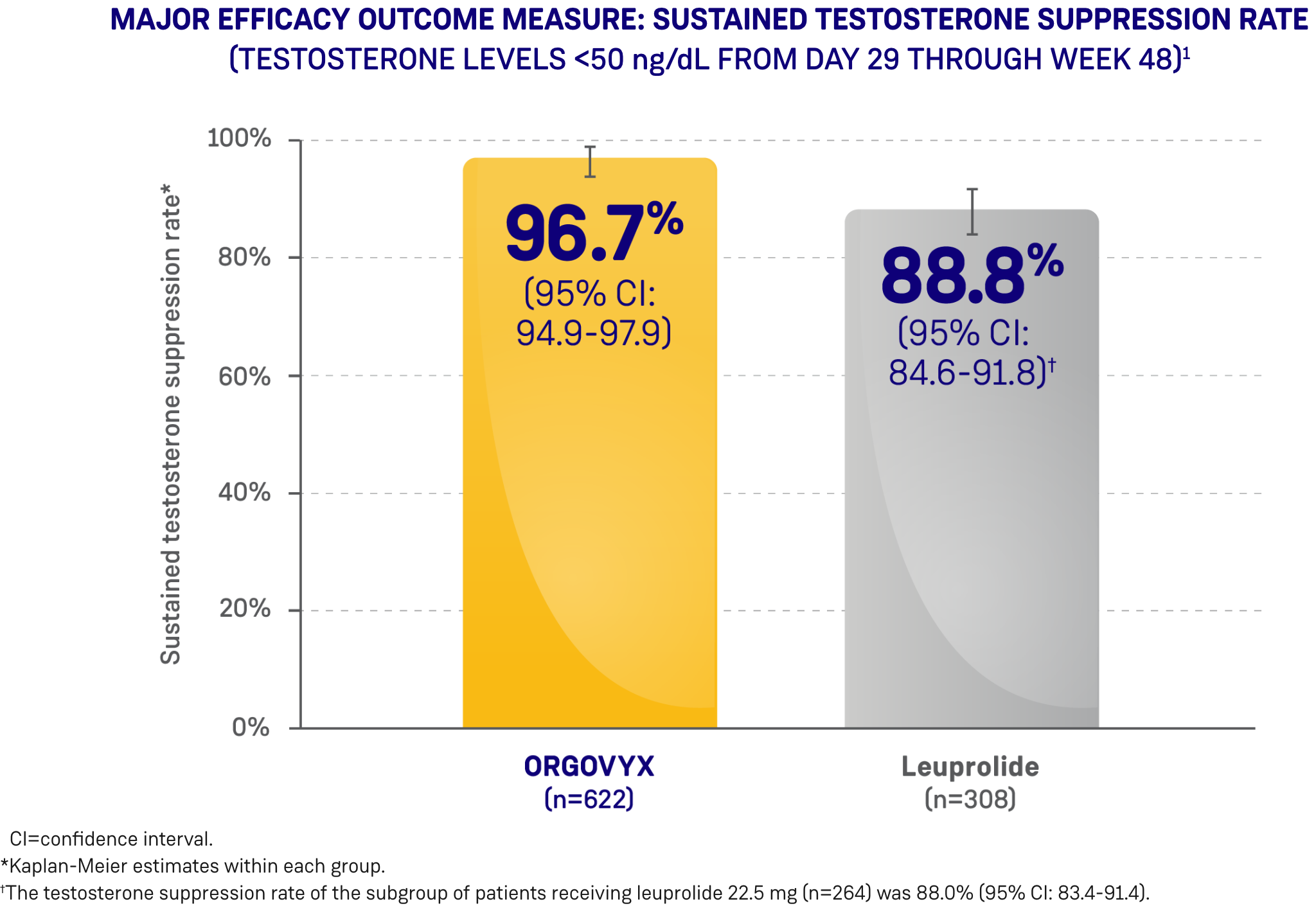


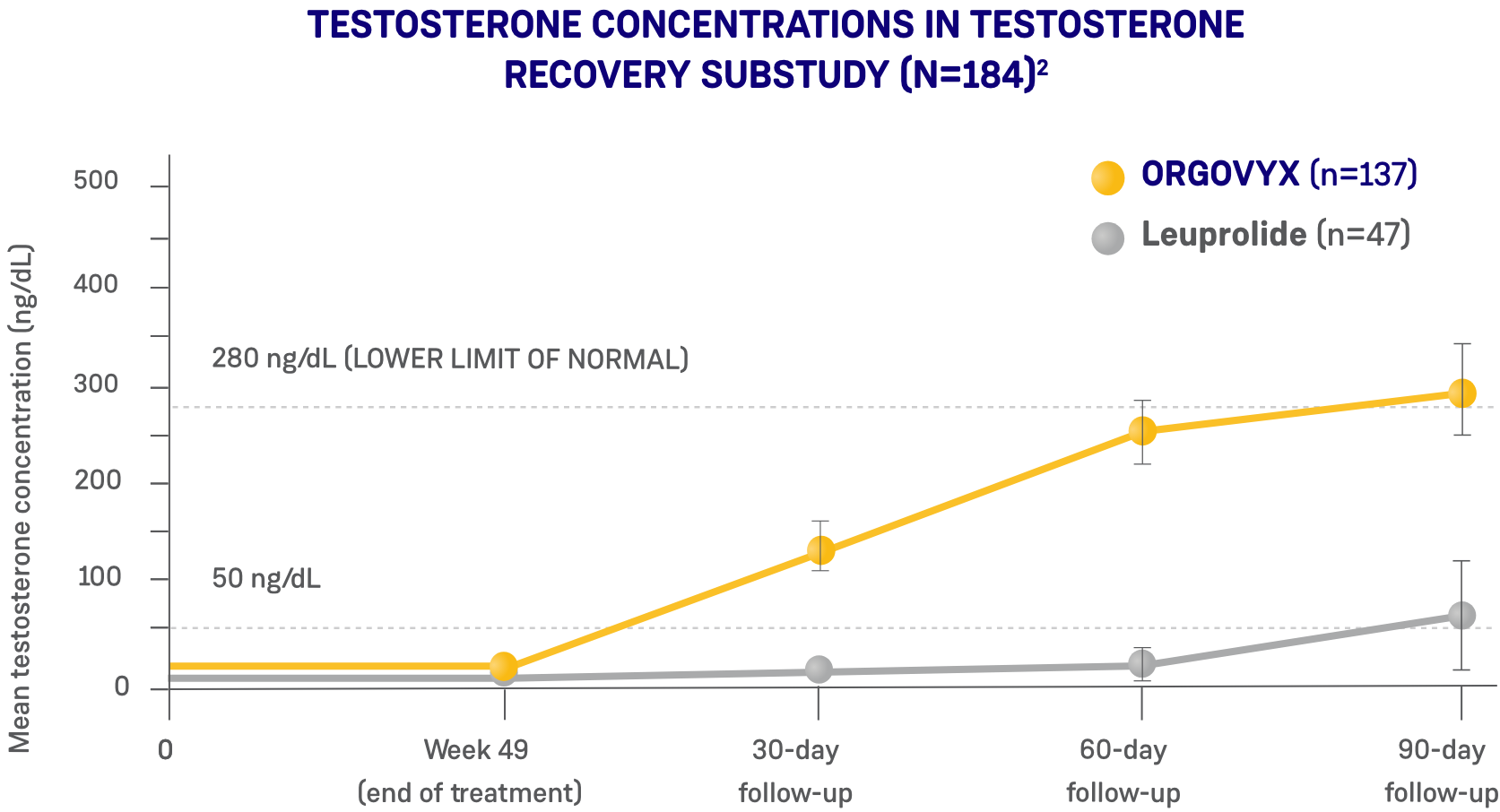
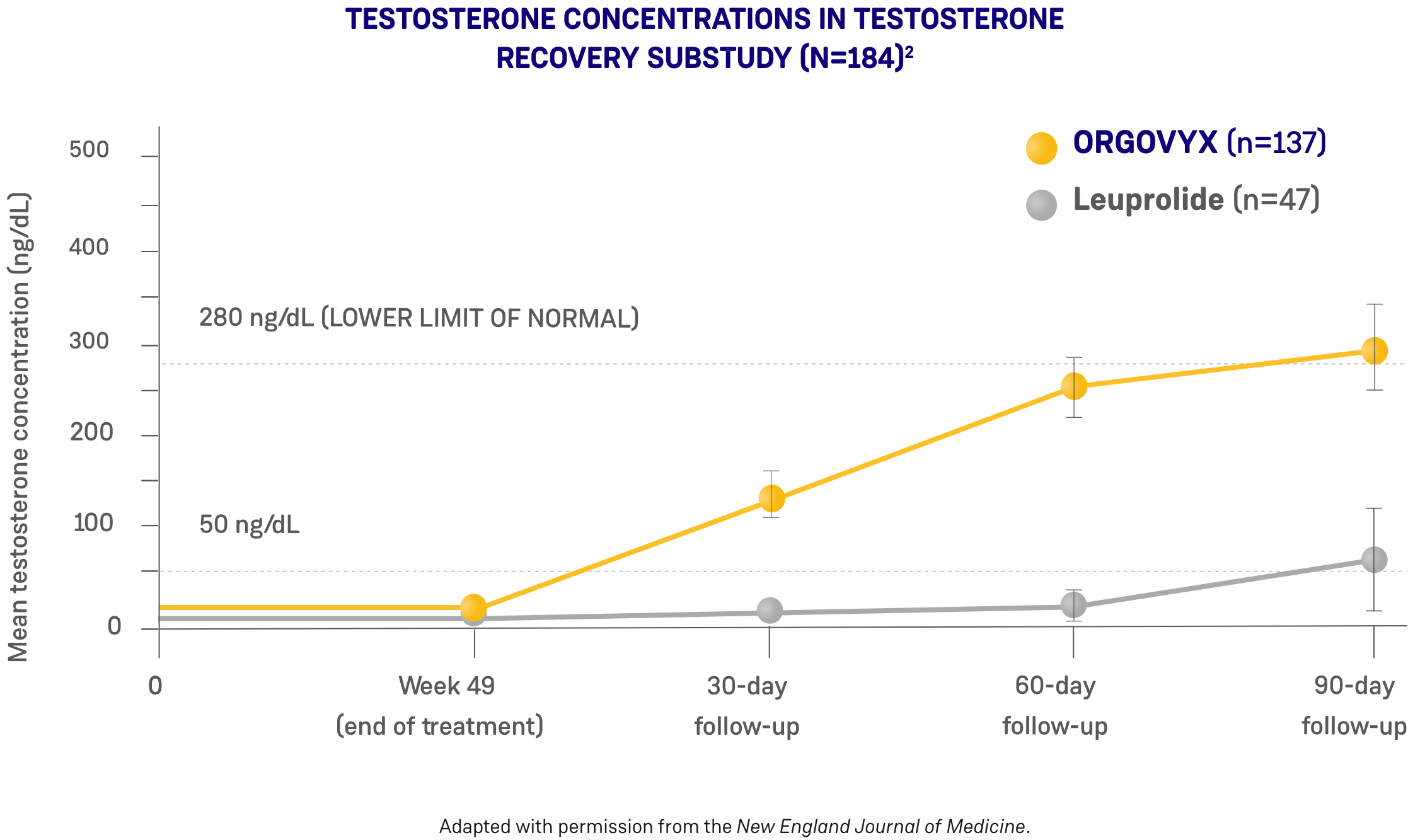
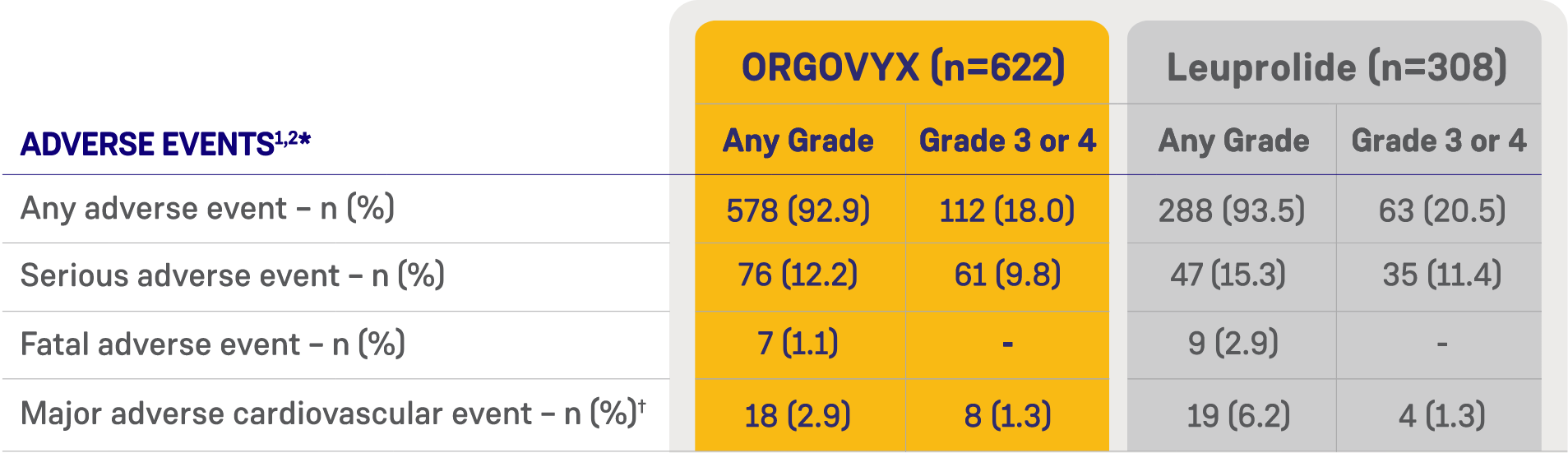
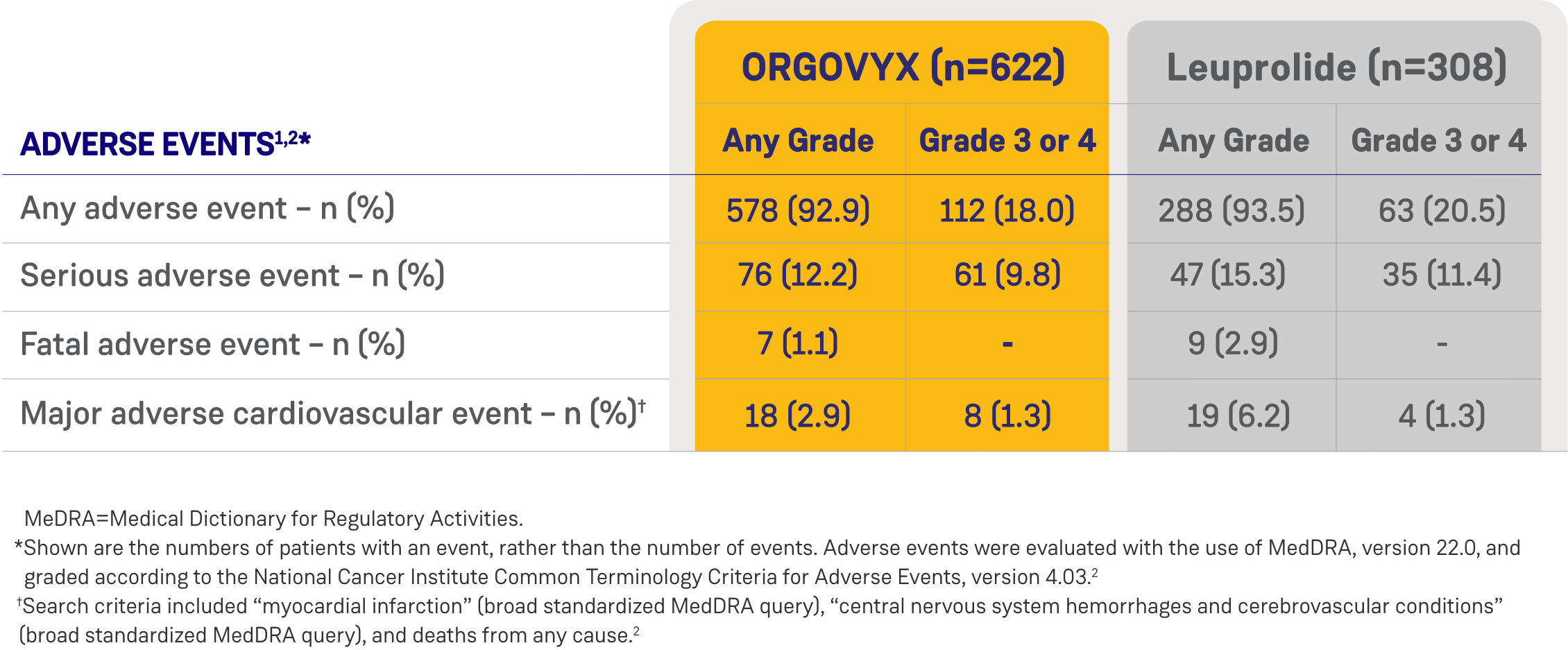


*ORGOVYX Copay Assistance Program: Terms and Conditions
The ORGOVYX Copay Assistance Program (“Copay Program”) is for eligible patients with commercial prescription insurance for ORGOVYX. With this Copay Program, eligible patients will pay as little as $10 per month, subject to a maximum of $10,000 per calendar year. After the annual maximum of $10,000 for ORGOVYX is reached, patient will be responsible for the remaining monthly out-of-pocket costs. This Copay Program may not be redeemed more than once per 21 days. The Copay Program is not valid for patients whose prescription claims are reimbursed, in whole or in part, by any state or federal government program, including, but not limited to, Medicaid, Medicare, Medigap, Department of Defense (DoD), Veterans Affairs (VA), TRICARE, Puerto Rico Government Insurance, or any state patient or pharmaceutical assistance program. Offer is not valid for cash-paying patients. Patient must be a resident of the U.S., Puerto Rico, or U.S. Territories. This Copay Program is void where prohibited by state law and on the date an AB generic equivalent for ORGOVYX becomes available. Certain rules and restrictions apply. This offer is not insurance. This offer cannot be combined with any other coupon, free trial, discount, prescription savings card, or other offer. This offer is not conditioned on any past, present, or future purchase, including refills. Patient and participating pharmacists agree not to seek reimbursement for all, or any part of the benefit received by the patient through this Copay Program. Patient and participating pharmacists agree to report the receipt of Copay Program benefits to any insurer or other third party who pays for or reimburses any part of the prescription filled using the Card, as may be required by such insurer or third party. Sumitomo Pharma America reserves the right to revoke, rescind, or amend this offer without notice. The ORGOVYX Copay Program is valid through December 31, 2024.
*ORGOVYX Bridge Program: Terms and Conditions
The ORGOVYX Bridge Program (“Bridge Program”) provides ORGOVYX at no cost for a limited period (up to 4 months) in a calendar year to eligible, commercially-insured patients, who have been prescribed ORGOVYX for an FDA-approved indication, and whose insurance coverage is delayed or who experience a temporary lapse in coverage. Prescribers must complete the Bridge Program prescription on the start form. By participating, patient acknowledges intent to pursue insurance coverage for ORGOVYX with their healthcare provider. Patients will receive their maintenance drug supply each month for up to 4 months or until they receive insurance coverage approval, whichever occurs earlier. The Bridge Program is not available for patients whose prescription claims are reimbursed, in whole or in part, by any state or federal government program, including, but not limited to, Medicaid, Medicare, Medigap, Department of Defense (DoD), Veterans Affairs (VA), TRICARE, Puerto Rico Government insurance, or any state patient or pharmaceutical assistance program. Patients must be residents of the United States or US Territories. The Bridge Program is not available to patients who are uninsured or where prohibited by law such as Massachusetts and Minnesota. Patients may be asked to reverify insurance coverage status during the course of the Bridge Program. Patients and participating prescribers agree not to seek reimbursement for all, or any part of the benefit received by the patient through this Bridge Program. No purchase necessary. The Bridge Program is not health insurance, nor is participation a guarantee of insurance coverage. Other limitations may apply. Sumitomo Pharma America reserves the right to rescind, revoke, or amend the Bridge Program and discontinue support at any time without notice.
References: 1. ORGOVYX (relugolix) [prescribing information]. Brisbane, CA: Sumitomo Pharma America; 2023. 2. Shore ND, Saad F, Cookson MS, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382(23):2187-2196 and supplementary material, available online. 3. Shore ND, Saad F, Cookson MS, et al. HERO phase 3 trial: relugolix, an oral GnRH receptor antagonist, versus leuprolide acetate for advanced prostate cancer. Presented at: American Society of Clinical Oncology Virtual Scientific Program; May 29-June 2, 2020; virtual. Abstract 5602. 4. Reyes C, Groshel C, Given R. Androgen deprivation therapy. In: Trabulsi EJ, Lallas CD, Lizardi-Calvaresi AE, eds. Chemotherapy and Immunotherapy in Urologic Oncology: A Guide for the Advanced Practice Provider. Springer International Publishing; 2021:77-92. 5. US Food and Drug Administration. FDA approves first oral hormone therapy for treating advanced prostate cancer. December 18, 2020. Accessed January 13, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-hormone-therapy-treating-advanced-prostate-cancer 6. Higano CS, Hafron J. Adherence with oral anticancer therapies: clinical trial vs real-world experiences with a focus on prostate cancer. J Urol. 2023;209(3):485-493. doi:10.1097/JU.0000000000003081 7. Fleshner NE, Alibhai SMH, Connelly KA, et al. Adherence to oral hormonal therapy in advanced prostate cancer: a scoping review. Ther Adv Med Oncol. 2023;15:1-22. doi:10.1177/17588359231152845 8. Lowrance WT, Breau RH, Chou R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline part Il. J Urol. 2021;205(1):22-29. doi:10.1097/JU.0000000000001376 9. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer V.4.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed November 14, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 10. Lowrance WT, Breau RH, Chou R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline part I. J Urol. 2021;205(1):14-21. doi:10.1097/JU.0000000000001375 11. Crawford ED, Heidenreich A, Lawrentschuk N, et al. Androgen-targeted therapy in men with prostate cancer: evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019;22(1):24-38. doi:10.1038/s41391-018-0079-0 12. Data on file. Sumitomo Pharma America. 13. Moul JW. The evolving definition of advanced prostate cancer. Rev Urol. 2004;6(suppl 8):S10-S17.

SUMITOMO PHARMA is a trademark of Sumitomo Pharma Co., Ltd., used under license.
SUMITOMO is a registered trademark of Sumitomo Chemical Co., Ltd., used under license.
Sumitomo Pharma America, Inc. is a U.S. subsidiary of Sumitomo Pharma Co. Ltd.
ORGOVYX® and its associated logo are registered trademarks of Sumitomo Pharma Switzerland GmbH.
©2023 Sumitomo Pharma Switzerland GmbH and Pfizer Inc. All rights reserved. PP-US-REL-2300278 12/2023

INDICATION
ORGOVYX® (relugolix) is a gonadotropin-releasing hormone (GnRH) receptor antagonist indicated for the treatment of adult patients with advanced prostate cancer.
IMPORTANT SAFETY INFORMATION
Contraindication
ORGOVYX is contraindicated in patients with severe hypersensitivity to relugolix or to any of the product components.
Warnings and Precautions
QT/QTc Interval Prolongation: Androgen deprivation therapy, such as ORGOVYX may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, or frequent electrolyte abnormalities and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes.
Hypersensitivity: Angioedema was reported in 0.2% of patients treated with ORGOVYX in HERO. Hypersensitivity reactions, including pharyngeal edema and other serious cases of angioedema, have been reported in post-marketing with ORGOVYX. Advise patients who experience any symptoms of hypersensitivity to temporarily discontinue ORGOVYX and promptly seek medical care. Discontinue ORGOVYX for severe hypersensitivity reactions and manage as clinically indicated.
Embryo-Fetal Toxicity: The safety and efficacy of ORGOVYX have not been established in females. Based on findings in animals and mechanism of action, ORGOVYX can cause fetal harm and loss of pregnancy when administered to a pregnant female. Advise males with female partners of reproductive potential to use effective contraception during treatment and for 2 weeks after the last dose of ORGOVYX.
Laboratory Testing: Therapy with ORGOVYX results in suppression of the pituitary gonadal system. Results of diagnostic tests of the pituitary gonadotropic and gonadal functions conducted during and after ORGOVYX may be affected. The therapeutic effect of ORGOVYX should be monitored by measuring serum concentrations of prostate-specific antigen (PSA) periodically. If PSA increases, serum concentrations of testosterone should be measured.
Adverse Reactions
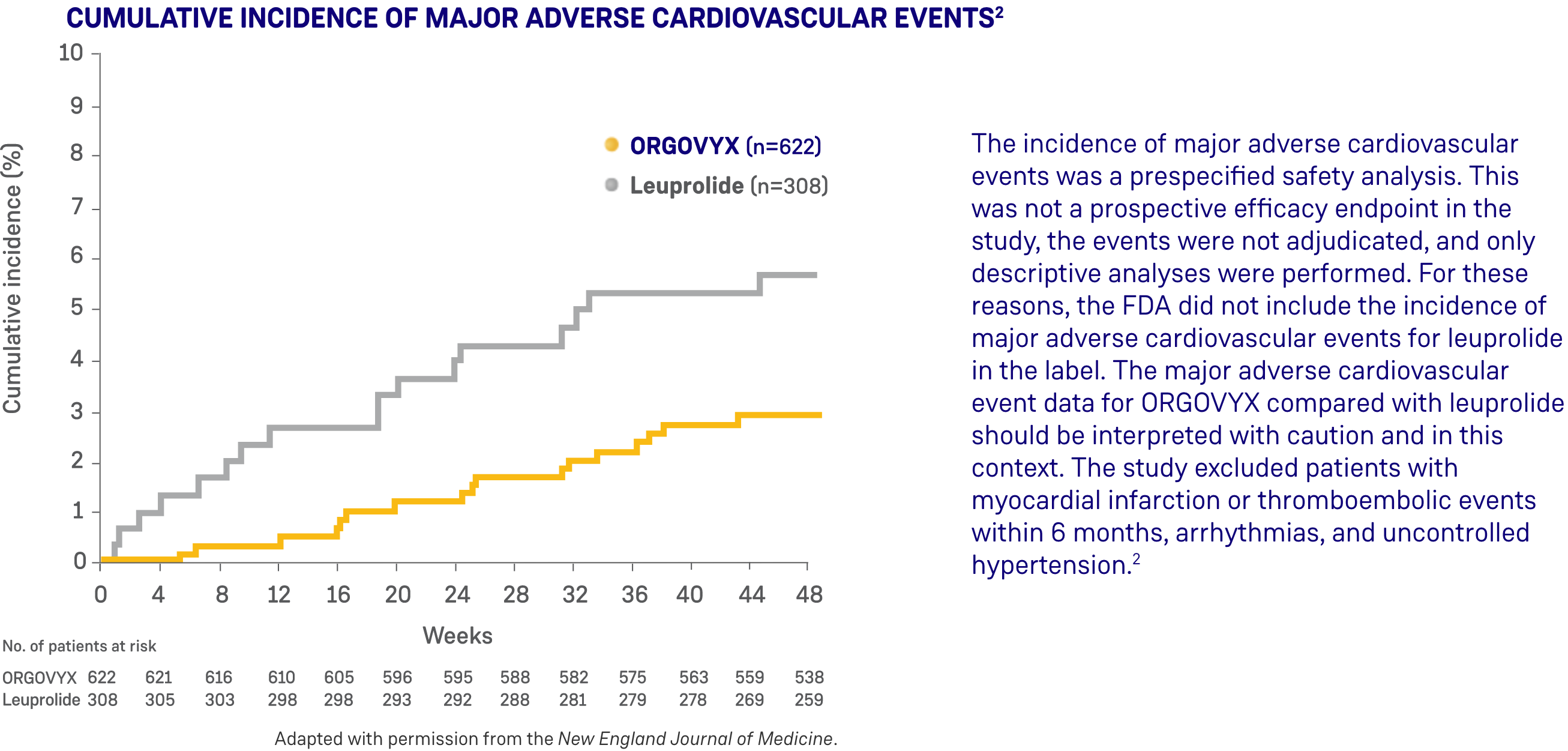
Serious adverse reactions occurred in 12% of patients receiving ORGOVYX. Serious adverse reactions in ≥0.5% of patients included myocardial infarction (0.8%), acute kidney injury (0.6%), arrhythmia (0.6%), hemorrhage (0.6%), and urinary tract infection (0.5%). Fatal adverse reactions occurred in 0.8% of patients receiving ORGOVYX including metastatic lung cancer (0.3%), myocardial infarction (0.3%), and acute kidney injury (0.2%). Fatal and non-fatal myocardial infarction and stroke were reported in 2.7% of patients receiving ORGOVYX.
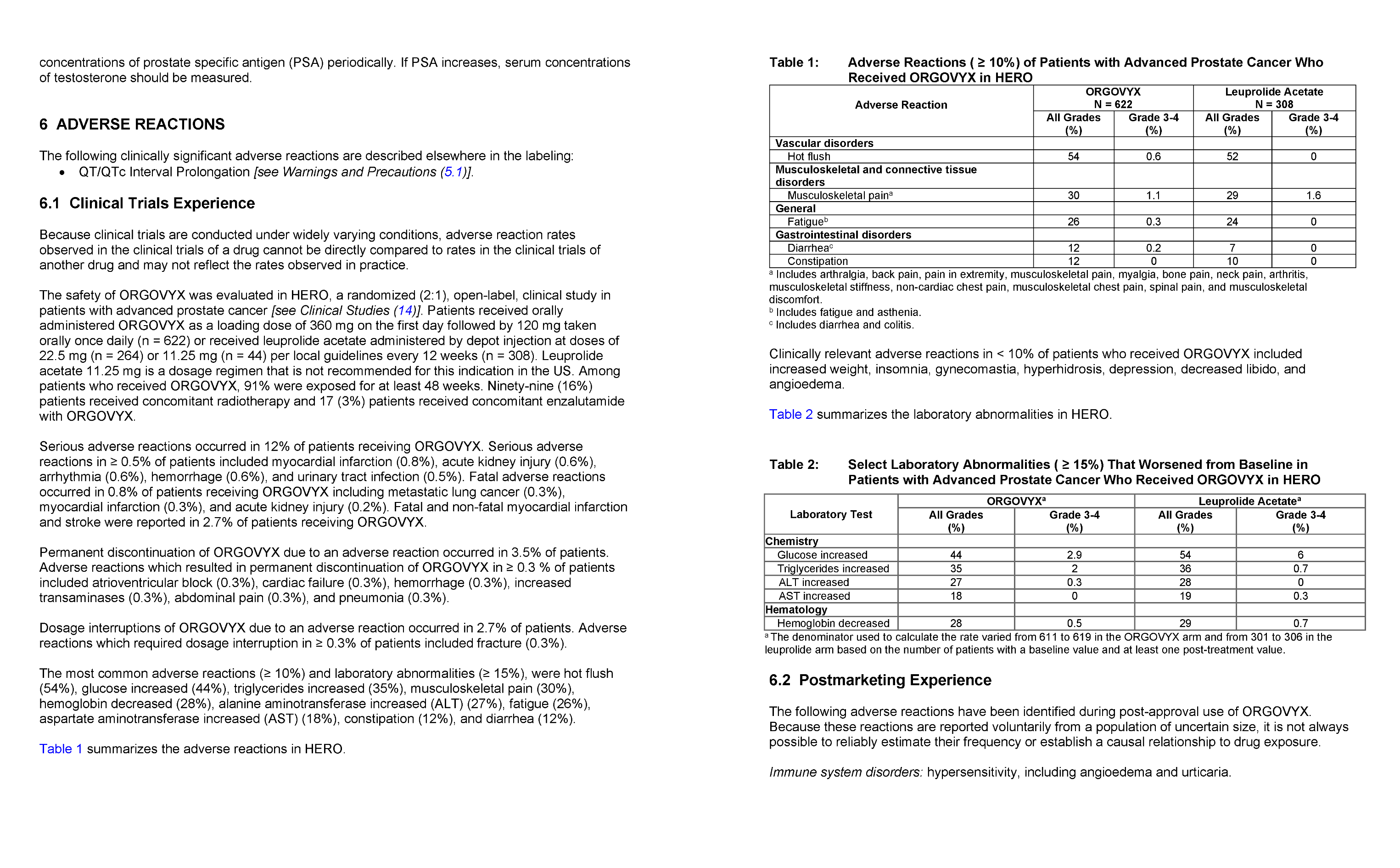
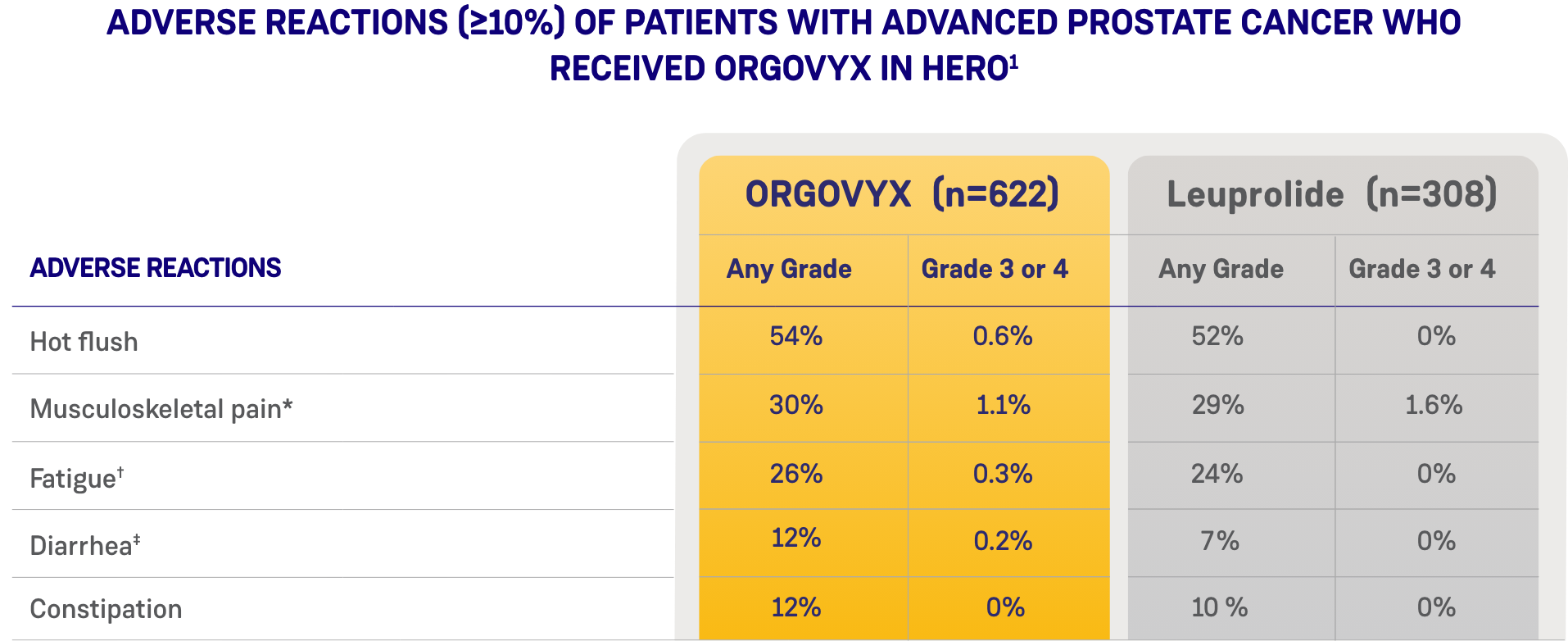
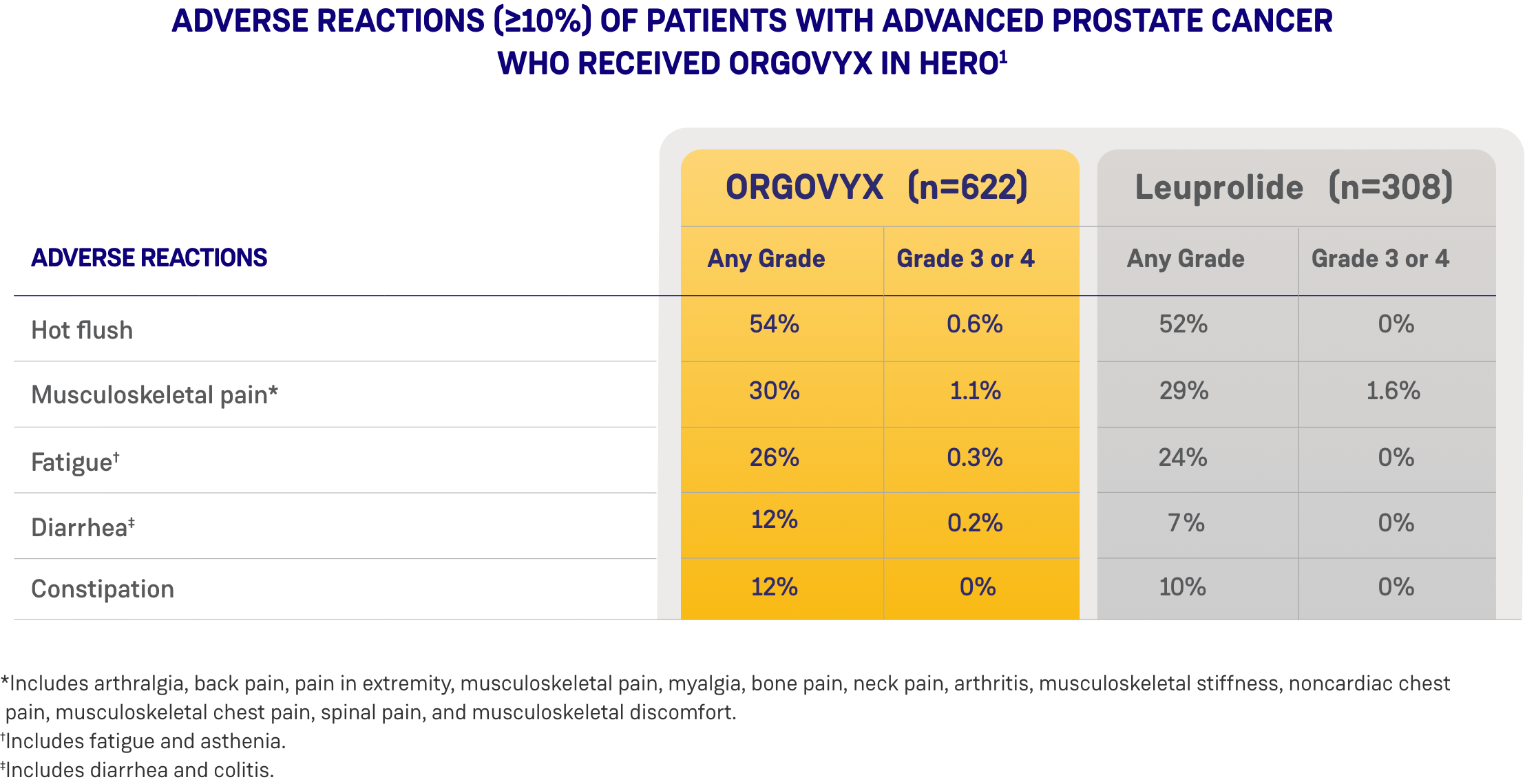
Most common adverse reactions (10%) and laboratory abnormalities (15%) in patients receiving ORGOVYX were hot flush (54%), glucose increased (44%), triglycerides increased (35%), musculoskeletal pain (30%), hemoglobin decreased (28%), alanine aminotransferase increased (27%), fatigue (26%), aspartate aminotransferase increased (18%), constipation (12%), and diarrhea (12%).
Drug Interactions
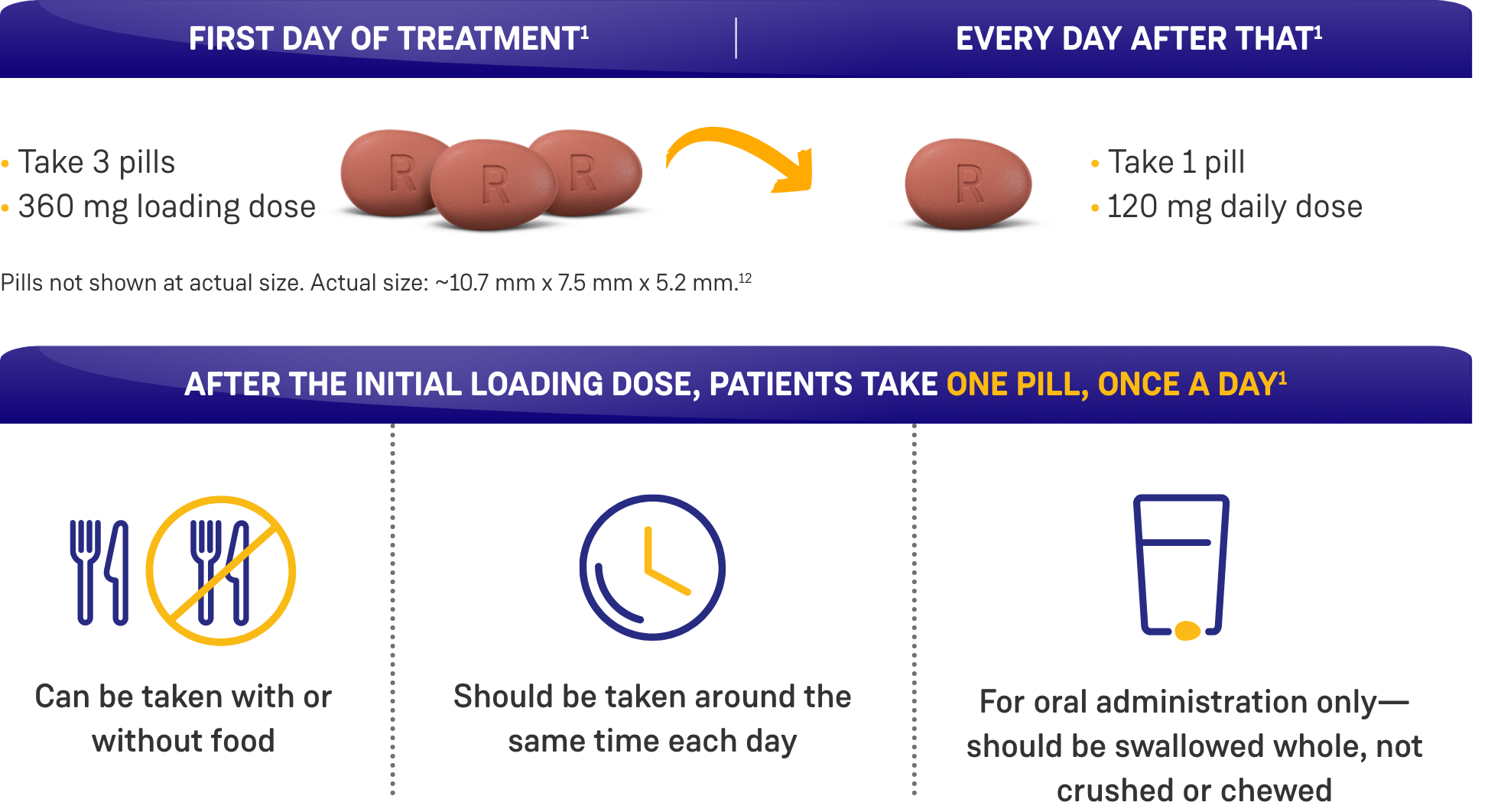
Co-administration of ORGOVYX with a P-gp inhibitor increases the area under the curve (AUC) and maximum concentration (Cmax) of ORGOVYX, which may increase the risk of adverse reactions associated with ORGOVYX. Avoid co-administration of ORGOVYX with oral P-gp inhibitors. If co-administration is unavoidable, take ORGOVYX first, separate dosing by at least 6 hours, and monitor patients more frequently for adverse reactions. Treatment with ORGOVYX may be interrupted for up to 2 weeks for a short course of treatment with certain P-gp inhibitors. If treatment with ORGOVYX is interrupted for more than 7 days, resume administration of ORGOVYX with a 360 mg loading dose on the first day, followed by 120 mg once daily.
Co-administration of ORGOVYX with a combined P-gp and strong CYP3A inducer decreases the AUC and Cmax of ORGOVYX, which may reduce the effects of ORGOVYX. Avoid co-administration of ORGOVYX with combined P-gp and strong CYP3A inducers. If co-administration is unavoidable, increase the ORGOVYX dose to 240 mg once daily. After discontinuation of the combined P-gp and strong CYP3A inducer, resume the recommended ORGOVYX dose of 120 mg once daily.